LGG® clinical evidence
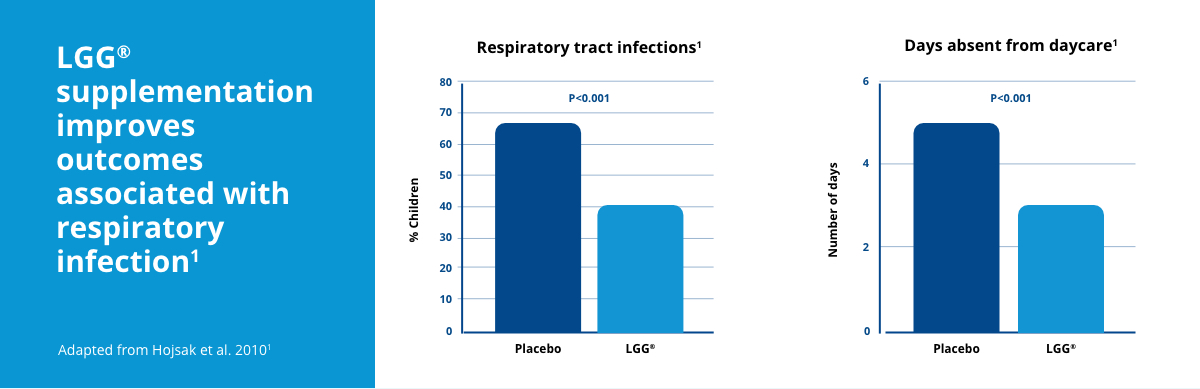
Daily intake of LGG® at 1 billion CFU over a 3 month period was associated with a significant reduction in the number of children with URTIs, the number of RTIs lasting longer than three days, and the duration of respiratory symptoms.1 Compared to children in the placebo group, children supplemented with LGG® also had significantly fewer days away from daycare due to illness.1
LGG® has also been associated with fewer hospital-acquired infections in children.16 When children admitted to hospital were supplemented with LGG® at 1 billion CFU/day in fermented milk for the length of their stay, there was a significant reduction in the number of gastrointestinal infections lasting longer than two days, and a significant reduction in the number of respiratory infections lasting longer than three days.16
Click to read more about the Chr. Hansen LGG® strain.
BB-12® clinical evidence
BB-12® has been associated with reduced incidence of respiratory infections in infants.17, 18 One-month old, healthy infants were supplemented with BB-12® at 10 billion CFU/day or with placebo until they were 8 months old. The infants supplemented with BB-12® had a significantly reduced risk of respiratory infections compared to placebo (65% vs 94%, P=0.014).17
Following the initial analysis, the same infants continued in their original treatment groups (placebo, or BB-12® at 10 billion CFU/day) until they were 2 years old. A similar effect was observed; the infants in the BB-12® group had significantly fewer RTIs than those in the placebo group. The risk of respiratory infections was 87% in the infants supplemented with BB-12® and 100% in placebo (P=0.033).18
Click to read more about the Chr. Hansen BB-12® strain.