Global guidelines
- probiotic recommendations for infants and children

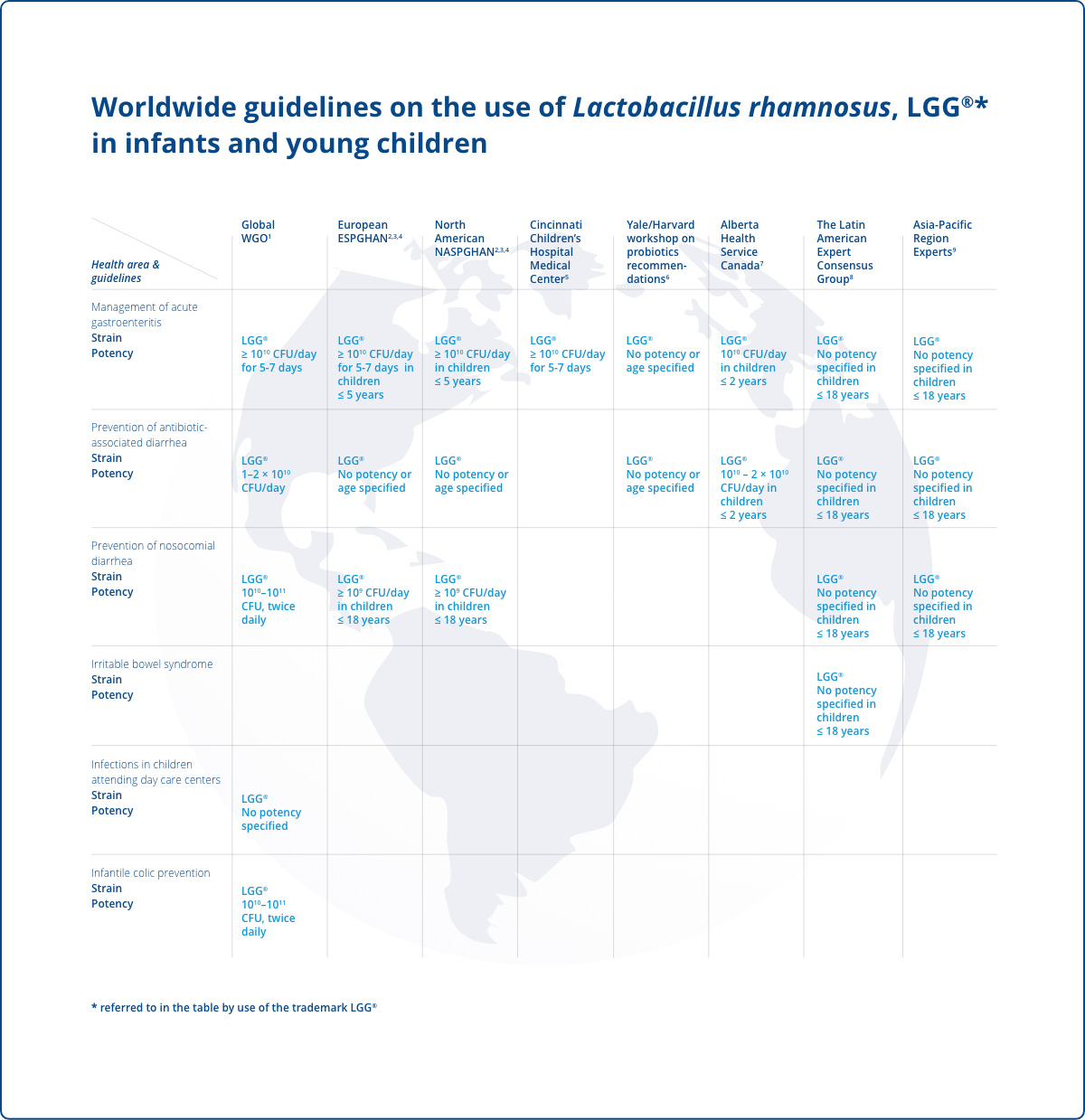
The World Gastroenterology Organization (WGO) propose the highest level of evidence for the use of the LGG® strain for:
- Treatment of acute gastroenteritis in a pediatric population (≥ 1010 CFU/day)1
- Prevention of antibiotic-associated diarrhea in a pediatric population (1–2 × 1010 CFU/day)1
- Prevention of nosocomial diarrhea in a pediatric population (1010–1011 CFU, twice daily)1
- Prevention of infections in children attending day-care centers (no dose specified)1
The WGO propose the second highest level of evidence for the use of the LGG® strain as:
- Adjuvant therapy in the treatment of Helicobacter pylori in an adult population (6 × 109, twice daily)1
The WGO propose the third level of evidence for the following combination of strains:
- BB-12®, Lactobacillus acidophilus, LA-5®, Lactobacillus delbrueckii subsp. Bulgaricus, LBY-27™, and Streptococcus thermophilus, STY-31™, for the treatment of irritable bowel syndrome in adults (4 × 109 CFU, twice daily)1
- BB-12® with Lactobacillus acidophilus, LA-5® for the treatment of non-alcoholic fatty liver disease in adults1
CFU = colony forming units
Europe
The European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) propose the LGG® strain for:
- Use against acute gastroenteritis (≥ 1010 CFU/day) in children ≤5 years2
- Antibiotic-associated diarrhea (no specified dose and age)3
- Prevention of nosocomial diarrhea (≥ 109 CFU/day) in those up to 18 years4
North America
The North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) propose the LGG® strain for:
- Use against acute gastroenteritis (≥ 1010 CFU/day) in children ≤ 5 years2
- Antibiotic-associated diarrhea (no specified dose and age)3
- Prevention of nosocomial diarrhea (≥ 109 CFU/day) in those ≤ 18 years4
The Cincinnati Children's Hospital Medical Center propose:
- Implementation of the LGG® strain for the treatment of acute diarrhea in children (1010 CFU/day)5
The Yale University/Harvard University joint workshop, propose the LGG® strain for the treatment of:
- Infectious diarrhea6
- Antibiotic-associated diarrhea6
- Prevention of antibiotic-associated diarrhea6
- Prevention and treatment of atopic eczema associated with cow’s milk allergy (no doses specified)6
Canada
The Alberta Health Service propose that the LGG® strain be used in infants and children under 2 years of age for:
- Treatment of acute gastroenteritis (1010 CFU/day)
- Prevention of antibiotic-associated diarrhea (1010 – 2 × 1010 CFU/day)7
Latin America
The Latin American Expert Consensus Group propose the LGG® strain in infants and children up to 18 years of age for:
- Prevention and treatment of acute infectious diarrhea8
- Prevention of nosocomial diarrhea, and prevention of antibiotic-associated diarrhea (no doses specified).8
- Symptoms of irritable bowel syndrome8
APAC region
An Asia Pacific Region expert group propose the use of the LGG® strain for:
- Treatment of acute gastroenteritis in children9
- Prevention of antibiotic-associated diarrhea in children9
- Nosocomial diarrhea in children (no doses specified)9
LGG® and BB-12® are registrered trademarks of Chr. Hansen A/S.
References Open Close
1. World Gastroenterology Organisation. World Gastroenterology Organisation Global Guidelines - Probiotics and prebiotics. 2017.
2. Guarino A, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132-52. (PubMed)
3. Szajewska H, et al. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Children. J Pediatr Gastroenterol Nutr. 2016;62(3):495-506. (PubMed)
4. Hojsak I, et al. Probiotics for the Prevention of Nosocomial Diarrhea in Children. J Pediatr Gastroenterol Nutr. 2018;66(1):3-9. (PubMed)
5. Parker MW, et al. Rapid adoption of Lactobacillus rhamnosus GG for acute gastroenteritis. Pediatrics. 2013;131 Suppl 1:S96-102. (PubMed)
6. Floch MH, et al. Recommendations for Probiotic Use--2015 Update: Proceedings and Consensus Opinion. J Clin Gastroenterol. 2015;49 Suppl 1:S69-73. (PubMed)
7. Alberta Health Services. Nutrition Guideline: Healthy Infants and Young Children - Prebiotics and Probiotics. 2017.
8. Cruchet A, et al. The use of probiotics in pediatric gastroenterology; a review of the literature and recommendations by Latin-American experts. Paediatr Drugs. 2015;17(3);199-216
9. Cameron D, et al. Probiotics for gastrointestinal disorders: Proposed recommendations for children of the Asia-Pacific region. World J Gastroenterol. 2017;23(45):7952-64. (PubMed)
Clinical
studies
Learn how to review the evidence when considering probiotic strains and effects
Our
strains
Read more about some of the world’s most documented probiotic strains and their diverse, beneficial health effects



